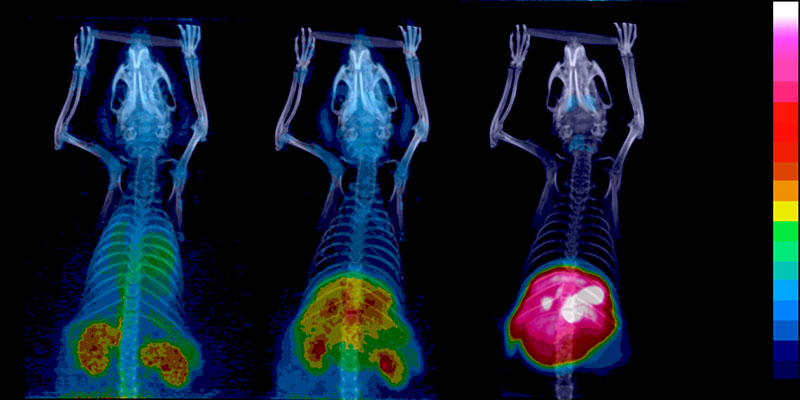
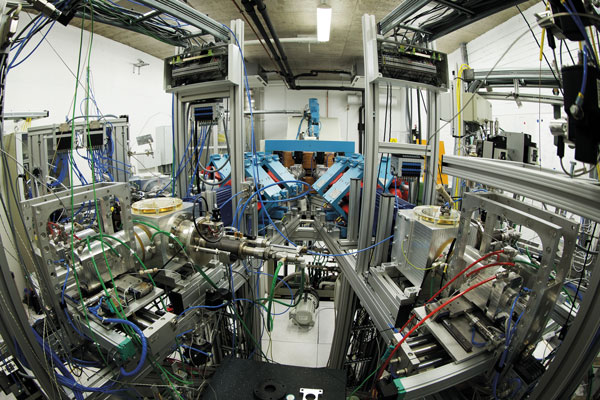
Positron emission tomograhy (PET) is a technique for visualisation which requires a vast infrastructure. In Turku, Åbo Akademi University, Turku University and Turku University Hospital are cooperating in PET activities and the development of a national PET Centre.
By Marcus Prest
The heart of the PET Centre, the particle accelerator – the cyclotron, is situated down in the basement of a stone building situated behind the university hospital main building, and it is hidden behind a seven tonne, radiation-safe steel door. The space is barred while the production of isotopes is going on. Production is kept up for five days a week and the isotopes are highly radioactive. They are used for research and in the field of medical research for, for instance, diagnosing cancer. This research is carried out on the floors above the accelerator laboratory.
The cyclotron is operated from a control panel which is being supervised by the laboratory engineer Stefan Johansson (Åbo Akademi University) and researcher Johan Rajander (Åbo Akademi University). The three isotopes produced are carbon-11, fluorine-18, and copper-64, in the main. The accelerator laboratory at Åbo Akademi University is responsible for the production of radioactive isotopes, whereas radiochemistry, which is the joining of the isotopes to molecules that take the isotopes to the correct receptors in the human body, is the responsibility of Turku University. The diagnoses are made by Turku University Hospital.
The cyclotron is a kind of particle accelerator, which with the help of magnetic fields and a high-frequency alternating current, creates highly energetic particles out of ions that are passed out of the magnetic field in a controlled way, building a beam of ions. The cyclotron and its system for the transportation of beams has been constructed by the Efremov Institute in St Peterburg, whereas the rest of the production system and the transportation lines have been made by the experts at the PET Centre.
“We develop and produce the target systems or the target chambers ourselves,” says Stefan Johansson.
The target chambers are massive cubicles made out of aluminium, with precisely measured aisles for the ion beam and space for the actual radiation targets. Åbo Akademi University has its own metal workshop where people work with the aluminium parts.
“The two products that we produce most are carbon-11 and fluorine-18. Carbon and fluorine require different kinds of radiation targets,” says Johan Rajander.
The process – a simplified version
The production and application of the isotope carbon-11 can be explained as follows: The cyclotron shoots out an ion beam, in this particular case consisting of protons, with an energy of 18 MeV (million electron volts) which is guided and focused onto a target where the beam hits a gas at high pressure; it is a mixed gas consisting of nitrogen and one per cent oxygen at a pressure of circa 40 atmospheres. The pressure is adapted to the length of the target – different pressures give different densities – which affects the range of the proton beam in the gas. As the protons hit the nitrogen, the beam diffuses, which explains the conic shape of the target chambers. Simultaneously a radioactive reaction is taking place: one proton enters the nucleus, one alpha particle leaves it. Carbon-11 is a radioactive isotope with a half-life of 20.4 minutes. The radiation done, the irradiated gas is sent up to the lead-lined cabinets in the clean space of the radiochemistry laboratory on the second floor, and the isotope is connected with the right kind of molecule and is ‘refined’ into a preparation that can be injected; a radiopharmaceutical. The radiopharmaceutical is given as an injection to a waiting patient, who is then sent on to a PET examination where the physician reads off the radiation originating from the tailored molecule with the PET scanner. Carbon-11 and fluorine-18 decompose through the positron emission; this process is a particular kind of so-called ‘radioactive beta decay’.

Sarita Forsback at her daily task of producing FDG. The isotopes are delivered from the accelerator bunker up to a hot cell (a lead-lined cabinet) on the second floor, to be manipulated so that they can be used for radiomedical purposes. Photo: Roni Lehti
“When the positrons, which originate from the radioactive decay, hit electrons, an annihilation occurs and two gamma quanta photons move in the opposite direction, which is an event that may be detected by means of a PET scanner. It’s a clear radioactive signal. You consequently get a good image,” says doctor Sarita Forsback from Turku University.
The radioactive preparations are short-lived pharmaceuticals. New ones have to be produced each day and in order for the right kind of product to be made at the right point in time, a great deal of Forsback’s time goes into ensuring that all the timetables cohere with the coordination of the accelerator’s operation and the work done in the lead-lined cabinets, and into making sure that the right patient is waiting when the preparation and the relevant staff are ready at the PET scanner.
“One can feel a strong sense of responsibility for the whole thing at this point,” says Forsback.
These preparations are not anything like Burana painkillers. The isotopes are radioactive and all of the work that is done with them must be done in lead-lined cabinets, and the quality of each round must be defined and tailored for the patient in question.
“Every new kind of radiopharmaceutical we have developed and every part of the system of the production process, must go through a meticulous process of checking before they are taken into use. FIMEA, which is the Finnish Medicines Agency, keeps us under constant scrutiny.”
The radioactive preparations are called pharmaceuticals despite the fact that they do not have any remedying effect. It is actually very important that they do not have an effect at all. The great advantage of radiochemistry is that it makes it possible to examine patients and decide the position of tumours, as well as whether the tumours are hypoxic (i.e., use oxygen, in which case radiation treatment is less effective), in organs which are difficult to reach, without having to use surgery. Deciding what kind of tumour is dealt with is also possible, to a certain extent, with the help of scanning, which means that it becomes possible to make more exact programmes for treatment.
“The molecules are tailored in such a way as to have a high affinity with a specific process in the human being. They gather in the receptors that we examine without affecting the function of the receptors. A large part of the development work we do within radiochemistry is about finding a way to make radioactive isotopes connect with the right kind of molecule.”
The radiopharmaceutical which is used the most in examinations using PET is FDG, fluorodeoxyglucose, which is produced by attaching the isotope fluorine-18 to a glucose molecule. FDG can be accessed commercially, but the preparation is produced by the PET centre in Turku itself.
“FDG works on most tumours, since tumours tend to use very fast energy – sugar, that is. This makes FDG, which is consequently a glucose analogue – a sugar with a radioactive isotope – go looking for the tumour which burns sugar more intensely than other tissues. As the concentration of FDG then will be larger in the tumour tissue, we will also notice more gamma radiation from the annihilation of positrons from the FDG, hitting the electrons in the tumour’s mass.”
“For radiochemical marking, we use a method that has been developed in Turku and which is unique in the world. It is based on an electric discharge which dissolves all the connections in the molecule. Instead of always getting a loaded fluorine-18 ion, we can also create positively-loaded fluorine-18 ions, which provide us with more opportunities for combination. In addition, the process is both fast, automated, and it often produces radiopharmaceuticals which have highly advantageous features for imaging.”
In the work on her doctoral thesis, Sarita Forsback has herself developed two new types of molecules that are connected with dopamine receptors. It is possible to use the molecules in, for instance, research into Parkinson’s disease.
The Turku PET Centre
The Turku PET Centre was established in the 1970s. The centre acquired the status of a national research institute in 1996. It produces and carries out research into short-lived isotopes that emit radiation. These activities are authorised for medical research into diagnosing patients by means of radiochemistry.
The core activities of the PET Centre are based on an agreement between Åbo Akademi University, Turku University and Turku University Hospital. All of the activities are located at the university campus and the hospital area. The centre consists of an accelerator laboratory, a chemistry laboratory for radiopharmacology, a preclinical examination laboratory, and the PET laboratory. The staff at the Centre numbers more than 110 people at the moment, including staff and researchers representing various research fields. The PET Centre has three cyclotrons, 19 hot cells (for the production of tracers), and six PET or PET/CT scanners and digital ultrasound systems.
The centre has two main tasks: the production of high quality research and the provision of diagnostic services for the entire country. Research at the PET Centre is concentrated in four main fields: the energy metabolism of the cell, neurotransmission, preclinical visualisation, and radiochemical research. There is active cooperation between the universities and the medical industry.